This is an incomplete question, here is a complete question.
The chemical reaction is:
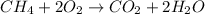
In the chemical reaction is 10 moles of H₂O are produced blank moles of CO₂ are produced.
Answer : The moles of CO₂ produced are, 5 moles.
Explanation : Given,
Moles of H₂O = 10 moles
The chemical reaction is:
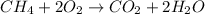
By the stoichiometry we can say that, 1 mole of CH₄ react with 2 mole of O₂ to give 1 mole of CO₂ and 2 moles of H₂O
From the balanced chemical reaction we conclude that,
As, 2 mole of H₂O produced with 1 mole of CO₂
So, 10 mole of H₂O produced with
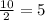 mole of CO₂
mole of CO₂
Thus, the moles of CO₂ produced are, 5 moles.