Answer:
Explanation:
Hello!
Using the information data of 204 endotermic reactions, two events were determined:
A: "the reactions final temperature is 271K or less"
B: "the heat absorbed is above target"
a) P(A∩B) is the intersection between the two events, this means, all the exotermic reaction with final temperature of 271K and less and absorved heat above target:
P(A∩B)=
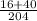 = 0.274
= 0.274
b)P(A∪B) is the probability that the reactions had a final temperature of 271K or are above target, the events A and B are not mutually exclusive, since both can occur at the same time (their intersection isn't void), so you calculate the probability as:
P(A∪B)= P(A) + P(B) - P(A∩B)
To calculate the probability of event A, you have to divide the number of reactions whose final temperature or less by the total number of endotermic reactions, symbolically:
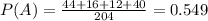
The probability of event B is the quotient between all reactions above target and the total of reactions, symbolically:
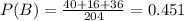
Then
P(A∪B)= 0.549 + 0.451 - 0.274= 0.726
c) P(A'∪B')
P(A'∪B')= P(A') + P(B') - P(A'∩B')
If A' is the complementary event of A, meaning all endotermic reactions above a final temperature of 271K, then it's probability is:
P(A')= 1 - P(A)= 1 - 0.549= 0.451
And B' the complementary event of B, this includes all reactions with heat absortion below target, it's probability is:
P(B')= 1 - P(B)= 1 - 0.451= 0.549
And the intersection between both events is:
P(A'∩B')= 56/204= 0.274
Then
P(A'∪B')=0.451 + 0.549 - 0.274= 0.726
I hope it helps!