Answer:
10 K
Step-by-step explanation:
Given that
Temperature rise of water = 10°C
Lets take initial temperature =T₁
Final temperature = T₂
Rise in temperature = T₂ - T₁
T₂ - T₁ = 10°C
We know that conversion of temperature unit is given as
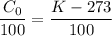
K= 273+ C
Initial temperature in kelvin =T₁ +273
Final temperature in kelvin =T₂ + 273
The increase in temperature = (T₂ + 273 )-(T₁ +273 ) = T₂ - T₁
Therefore the increase in temperature in Kelvin will be 10 K.