Answer:
[NO] = 0.636 M
Step-by-step explanation:
The equation for the reaction = N₂(g) + O₂(g) ⇄ 2NO(g)
Given that [N₂]=[O₂]= 0.300 M and [NO]=0.500 M
The equilibrium constant
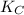 for the above equation can be written as:
for the above equation can be written as:
![K_(C)= ([NO]^2)/([N_2][O_2])](https://img.qammunity.org/2021/formulas/chemistry/college/10nb3j7gggdej2jadztcsb6pdyshzhjzd6.png)
(since there are 2 moles of NO and 1 mole of N₂ and O₂ in the equation above).
![K_(C)= ([0.500]^2)/([0.300][0.300])](https://img.qammunity.org/2021/formulas/chemistry/college/pih20brbmjjhrh45m2c4bnxl0vagd020zi.png)
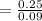
= 2.778
Furthermore, the question proceeds by stating that when more NO is added, there is increase in concentration, bringing it to 0.800 M.
Let construct an ICE table in order to determine the change in concentration
N₂(g) + O₂(g) ⇄ 2NO(g)
Initial (M) 0.300 0.300 0.800
Change (M) + x + x -2x
Equilibrium (M) 0.300+x 0.300+x 0.800-2x
Again,
![K_(C)= ([NO]^2)/([N_2][O_2])](https://img.qammunity.org/2021/formulas/chemistry/college/10nb3j7gggdej2jadztcsb6pdyshzhjzd6.png)
![2.778 = ([0.800-2x]^2)/([0.300+x][0.300+x])](https://img.qammunity.org/2021/formulas/chemistry/college/83xwnz6xms80anjactigk7fwu6gwcdh951.png)
![2.778 = ([0.800-2x]^2)/([0.300+x]^2)](https://img.qammunity.org/2021/formulas/chemistry/college/8umksc1oacrqum94nfltyqlw846zqciwqj.png)
Squaring both the Left-hand side(L.H.S) and the Right-hand side(R.H.S), we have:
![1.667=([0.800-2x])/([0.300+x])](https://img.qammunity.org/2021/formulas/chemistry/college/k59breofa4xhsuy8olmzy7afs9z8hlk9nq.png)
1.667[0.300+x] = [0.800-2x]
0.5001 + 1.667x = 0.800 - 2x
0.5001 - 0.800 = -1.667x - 2x
- 0.2999 = -3.667x
x =
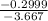
x = 0.082
So, the final concentration of NO from the ICE table after the equilibrium has been re-established is;
[NO] = 0.800 - 2x
[NO] = 0.800 - 2(0.082)
[NO] = 0.636 M
Therefore, the final concentration of NO be after equilibrium is re‑established = 0.636 M