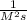Answer:
B.
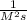
Step-by-step explanation:
The unit for rate is M/s while the unit for each molecule should be M. You can find the unit for k by putting the units for rate and the molecules into the equation
rate= k{X][Y]
M/s= k *
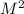 *
*
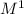
k= (M/s) / (
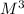 )
)
k=
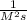
You can also use this predetermined formula to solve this problem faster: k=
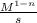
Where n is the number of molecule. There are 3 molecule(2X and 1Y) so n=3, so
k=
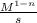
k=
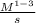 =
=
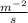 =
=