Here is the complete question.
Glycerol (C3H8O3), also called glycerine, is widely used in the food and pharmaceutical industries. Glycerol is polar and dissolves readily in water and polar organic solvents like ethanol. Calculate the mole fraction of the solvent in a solution that contains 1.61 g glycerol dissolved in 22.60 mL ethanol (CH3CH2OH; density = 0.7893 g/mol). Round to four significant digits
Answer:
0.9567 mol
Step-by-step explanation:
Given that:
mass of glycerol = 1.61 g
molar mass of glycerol = 92.1 g/mol
no of mole =

∴ number of moles of glycerol (
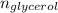 ) =
) =
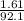
= 0.0175 mol
Volume of ethanol = 22.60 mL
Density of ethanol = 0.7893 g/mL
Since Density =

∴ mass of ethanol = density of ethanol × volume of ethanol
mass of ethanol = 0.7893 g/mL × 22.60 mL
mass of ethanol = 17.838 g
Number of moles of ethanol
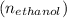 =
=
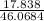
= 0.387 mole
∴ the mole fraction of the solvent can be determined as:
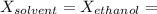
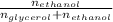
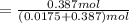
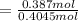
= 0.95673671199
≅ 0.9567 mol
∴ The mole fraction of the solvent in a solution that contains 1.61 g glycerol dissolved in 22.60 mL ethanol is = 0.9567 mol