Answer:
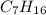
Step-by-step explanation:
Water is a polar solvent and hence can dissolve only polar solutes. Positive end of water attracts negative end of the solute and likewise negative end of water attracts positive end of the solute. This opposite attractions lead to dissolution.
In case of organic compound, water dissolve solute by forming hydrogen bond also.
Among the given, only
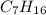 is non-polar and cannot form hydrogen bond with water too.
is non-polar and cannot form hydrogen bond with water too.
Therefore, only
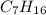 is not expected to dissolve in water.
is not expected to dissolve in water.