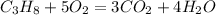The required balanced equation:
Step-by-step explanation:
• Propane when reacts with oxygen it gives carbon dioxide and water.
- In order to make the equation balanced at both ends of the equation,
Add 4 as a coefficient before on L.H.S (Left Hand Side) of Equation.
• Therefore, the required balanced equation is:
Propane, when added with 5 molecules of Water, yields 3 molecules of Carbon Dioxide and 4 molecules of water, as shown in the below equation -