Answer : The concentration to a mole fraction in parts per billion (ppb) is, 350 ppb
Explanation :
As we are given that the concentration of chloroform is,
 .
.
Now we have to convert it into parts per billion (ppb).
As we know that,
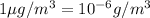
and,
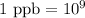
So,
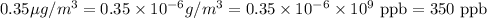
Thus, the concentration to a mole fraction in parts per billion (ppb) is, 350 ppb