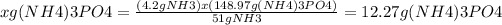Answer:
The answer is 12.27 g (NH4)3PO4
Step-by-step explanation:
Step 1: balance the chemical equation:
H3PO4 + 3NH3 → (NH4)3PO4
step 2: the molar masses of each of the reagents and products are calculated using the periodic table:
H3PO4:
3 atoms of H: 3x1=3 g/mol
1 atom of P: 1x30.97=30.97 g/mol
4 atoms of O: 4x16=64 g/mol
Molar mass=3+30.97+64=97.97 g/mol
3NH3:
3 atoms of N: 3x14=42 g/mol
9 atoms of H: 9x1=9 g/mol
Molar mass=42+9=51 g/mol
(NH4)3PO4:
3 atoms of N: 3x14=42 g/mol
12 atoms of H: 12x1=12 g/mol
1 atom of P: 1x30.97=30.97 g/mol
4 atoms of O: 4x16=64 g/mol
Molar mass=42+12+30.97+64=148.97 g/mol
we make a rule of three to calculate the amount of ammonium phosphate:
51 g NH3------------------148.97 g (NH4)3PO4
4.2 g NH3-----------------x g (NH4)3PO4
Clearing the x, we have: