Answer:
Decreasing order of melting point:
SrS >
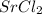 > RbCl > CsBr
> RbCl > CsBr
Step-by-step explanation:
Born–Landé equation explained the relation between lattice energy, charges and sizes of ions present in a compound.
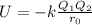
Here, U is internal energy,
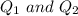 are charges and
are charges and
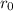 is the size.
is the size.
More the lattice energy more will be the energy to break ionic lattice in order to melt.
Therefore, more the lattice energy, more will be melting point.
Moreover, lattice energy is proportional to charge and inversely proportional to size.
Consider the compounds given
In
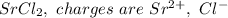
In CsBr, charges are
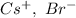
In RbCl charges are
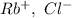
In Srs, charges on ions are
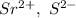
Order of the sizes are as follows:
Cs > Rb
Br > Cl
Cs > Sr
Therefore, decreasing order of melting point:
SrS >
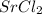 > RbCl > CsBr
> RbCl > CsBr