Answer: 3.306 mmol
Step-by-step explanation:
Molarity of a solution is defined as the number of moles of solute dissolved per Liter of the solution.
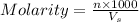
where,
Molarity
n= moles of solute
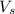 = volume of solution in ml
= volume of solution in ml
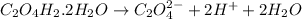
According to stoichiometry:
1 mole of
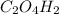 gives 2 moles of
gives 2 moles of

1.653 mmol of
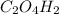 gives =
gives =
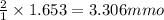 of
of

Thus number of millimoles of
 are 3.306
are 3.306