Answer:
The answer is 1.28 liters
Step-by-step explanation:
The solution to the question involves the use of Charles's Law of gasses which is also know as Gay-Lussac's Law. To get a bit of a context in understanding how the law works, imagine a balloon. The balloon is filled with air and is kept inside a freezer. As the balloon continues to cool, the air inside it also gets cooler which makes the balloon shrink. When the balloon is taken out of the fridge (assuming the same pressure and that the balloon has no holes of course), the balloon will start expanding again as the air inside it starts warming up. To explain this phenomenon, Charles' Law was developed which postulates that for a given level of pressure, the volume of a gas is proportional to its temperature. Note that the temperature referred to in the law is measured in Kelvin. This happens because the idea is that for a volume of zero (i.e no space is occupied) the temperature must be zero as well (lack of any heat). Since temperature can be measured in different units (e.g Celsius of Fahrenheit), it needs to be converted into Kelvin (zero Kelvin is actually -273.15 Celsius). So to convert Celsius into Kelvin you need to add 273.15
So, the equation of Charle's law is stated as
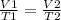
In the given example,
V1 = 1 Liter
T1 = -30 + 273.15 = 243.15 Kelvin
V2 = this is what we need to find
T2 = 37 + 273.15 = 310.15 Kelvin
So, our equation becomes V2 = V1 ×
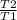 = 1 ×
= 1 ×
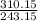 = 1.2755 ≅ 1.28 Liters
= 1.2755 ≅ 1.28 Liters
Therefore, the answer is 1.28 Liters