Answer: The given question is incomplete. The complete question is:
At a certain temperature the rate of this reaction is second order in
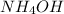 with a rate constant of
with a rate constant of
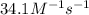 .
.
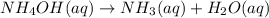
Suppose a vessel contains
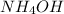 at a concentration of 0.100 M Calculate how long it takes for the concentration of
at a concentration of 0.100 M Calculate how long it takes for the concentration of
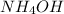 to decrease to 0.0240 M. You may assume no other reaction is important. Round your answer to 2 significant digits.
to decrease to 0.0240 M. You may assume no other reaction is important. Round your answer to 2 significant digits.
Answer: It takes 0.93 seconds for the concentration
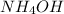 to decrease to 0.0240 M.
to decrease to 0.0240 M.
Step-by-step explanation:
Integrated rate law for second order kinetics is given by:
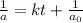
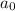 = initial concentration = 0.100 M
= initial concentration = 0.100 M
a= concentration left after time t = 0.0240 M
k = rate constant =
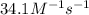
t = time taken for decomposition = ?
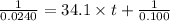
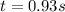
Thus it takes 0.93 seconds for the concentration
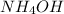 to decrease to 0.0240 M.
to decrease to 0.0240 M.