Question:-
A solution has a pH of 5.4, the determination of [H+].
Given :-
- pH:- 5.4
- pH = - log[H+]
To find :- concentration of H+
Answer:- Antilog(-5.4) or 4× 10-⁶
Explanation:-
Formula:- pH = -log H+
Take negative to other side
-pH = log H+
multiple Antilog on both side
(Antilog and log cancel each other )
Antilog (-pH) = [ H+ ]
New Formula :- Antilog (-pH) = [+H]
Now put the values of pH in new formula
Antilog (-5.4) = [+H]
we can write -5.4 as (-6+0.6) just to solve Antilog
Antilog ( -6+0.6 ) = [+H]
Antilog (-6) × Antilog (0.6) = [+H]
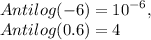
put the value in equation
![{10}^( - 6) * 4 = [H+] \\ 4 * {10}^( - 6) = [H+]](https://img.qammunity.org/2023/formulas/chemistry/college/w6bv9ouql5fu40xzvy34iihwkjk2w80jf2.png)