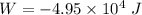Answer:
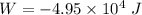
Step-by-step explanation:
given,
In first case Volume remains constant.
Work done in the first case is zero.
In Second case Volume change
V₁ = 0.2 m³
V₂ = 0.11 m³
Pressure, P = 5.5 x 10⁵ Pa
Work done = Pressure x change in volume
W = P ΔV
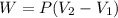
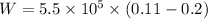
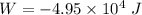
Hence, Work done when volume changes is equal to