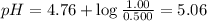Answer:
5.06
Step-by-step explanation:
Considering the Henderson- Hasselbalch equation for the calculation of the pH of the buffer solution as:
pH=pKa+log[base]/[acid]
Where Ka is the dissociation constant of the acid.
Given that the acid dissociation constant = 1.75×10⁻⁵
pKa = - log (Ka) = - log (1.75×10⁻⁵) = 4.76
Given concentration of acid = [acid] = 0.500 M
[Base] = 1.00 M