This is an incomplete question, here is a complete question.
A solution contains a mixture of pentane and hexane at room temperature. The solution has a vapor pressure of 270 torr . Pure pentane and hexane have vapor pressures of 425 torr and 151 torr, respectively, at room temperature.
What is the mole fraction of hexane?
Answer : The mole fraction of hexane is, 0.566
Explanation :
According to Raoult's law,
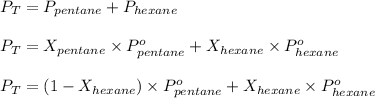
where,
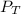 = total vapor pressure = 270 torr
= total vapor pressure = 270 torr
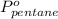 = vapor pressure of pure pentane = 425 torr
= vapor pressure of pure pentane = 425 torr
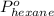 = vapor pressure of pure hexane= 151 torr
= vapor pressure of pure hexane= 151 torr
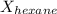 = mole fraction of hexane = ?
= mole fraction of hexane = ?
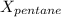 = mole fraction of pentane
= mole fraction of pentane
Now put all the given values in the above formula, we get:
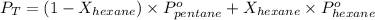
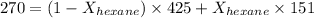
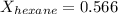
Thus, the mole fraction of hexane is, 0.566