Answer:
B. Steam burns the skin worse than hot water because the latent heat of vaporization is released as well.
Step-by-step explanation:
It is given that both steam and the boiling water when in contact with the skin cools down from 100 to 34 degrees Celsius.
For any substance of mass m, the heat required to change the temperature by
 is
is
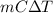 (S.I. unit = Joules).
(S.I. unit = Joules).
where C, the specific heat capacity is the same and a constant for both the condensed steam and the boiling water.
But, there is a "hidden" energy (heat) released by the steam called latent heat
(given by mL, L = specific latent heat) which allows the phase transition (gas to liquid). While both of them are at the same temperature, their energy (heat) is different, which is why steam causes burns worse than boiling water