Answer:
0.13 m of
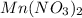 → Highest boiling point
→ Highest boiling point
0.19 m of
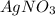 → Second Highest boiling point
→ Second Highest boiling point
0.17 m of
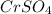 → Third highest boiling point
→ Third highest boiling point
0.31 m Sucrose (nonelectrolyte) → Lowest boiling point
Step-by-step explanation:
Elevation in boiling is given by :
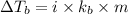
Where :
i = van't Hoff factor
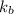 = Molal Elevation constant of solvent
= Molal Elevation constant of solvent
m = molaity of the solution
1) 0.19 m of
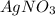
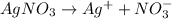
i = 2 (electrolyte)
Molality of the solution = 0.19
Elevation is boiling point of solution:
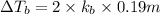
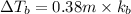
2) 0.17 m of
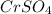
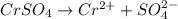
i = 2 (electrolyte)
Molality of the solution = 0.17
Elevation is boiling point solution :
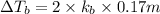
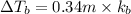
3) 0.13 m of
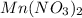
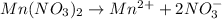
i = 3 (electrolyte)
Molality of the solution = 0.13
Elevation is boiling point solution :
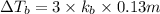
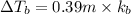
4) 0.31 m Sucrose (nonelectrolyte)
i = 1 ( non electrolyte)
Molality of the solution = 0.31 m
Elevation is boiling point solution :
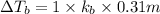

Higher the value of elevation in temperature higher will be the boiling point of the solution .
The decreasing order of solution from highest boiling point to lowest boiling point is :
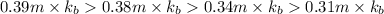
0.13 m of
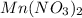 → Highest boiling point
→ Highest boiling point
0.19 m of
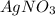 → Second Highest boiling point
→ Second Highest boiling point
0.17 m of
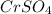 → Third highest boiling point
→ Third highest boiling point
0.31 m Sucrose (nonelectrolyte) → Lowest boiling point