Answer:
Concentration of
![[Fe^(3+)]](https://img.qammunity.org/2021/formulas/chemistry/college/9vyb68ikaq2mwz5wpztbxb86no4ye8wk3u.png) at equilibrium is 7.394 M.
at equilibrium is 7.394 M.
Step-by-step explanation:
Moles of ferric nitrate = 0.026 mol
Concentration of ferric nitrate =
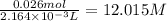
1 mole of ferric nitrate gives 1 mole of ferric ions, then 12.015 M of ferric nitrate solution will have:
[Fe^{3+}]=12.015 M[/tex]
Moles of
![Fe(SCN)]^(2+)](https://img.qammunity.org/2021/formulas/chemistry/college/gsafyzslnohhdbecirfzxbm2c2nw14vhci.png) at equilbrium = 0.01 mol
at equilbrium = 0.01 mol
Concentration of
![Fe(SCN)]^(2+)](https://img.qammunity.org/2021/formulas/chemistry/college/gsafyzslnohhdbecirfzxbm2c2nw14vhci.png) at equilbrium =
at equilbrium =
![[Fe(SCN)]^(2+)=(0.01 mol)/(2.164* 10^(-3) L)=4.621 M](https://img.qammunity.org/2021/formulas/chemistry/college/9qn0kgc2lraz016tjjbs522morhyzvkss6.png)
![Fe^(3+)+SCN^-\rightleftharpoons [Fe(SCN)]^(2+)](https://img.qammunity.org/2021/formulas/chemistry/college/2ba2px9nhf15q7skyq1k73vl47xcucphw2.png)
Initially
12.015 M 0
At equilibrium
(12.015 - 4.621 )M 4.621 M
Concentration of
![[Fe^(3+)]](https://img.qammunity.org/2021/formulas/chemistry/college/9vyb68ikaq2mwz5wpztbxb86no4ye8wk3u.png) at equilibrium
at equilibrium
= (12.015 - 4.621 )M = 7.394 M