Answer : The mass of arsenic present in 5.0 g of orpiment is, 1.5 grams.
Explanation : Given,
Mass of orpiment = 5.0 g
As we know that the molar mass of
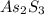 is, 246.02 g/mol and the molar mass of As is, 75 g/mol.
is, 246.02 g/mol and the molar mass of As is, 75 g/mol.
As per the question, we conclude that:
As, 246.02 g of
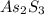 contains mass of arsenic = 75 g
contains mass of arsenic = 75 g
So, 5.0 g of
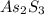 contains mass of arsenic =
contains mass of arsenic =
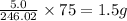
Thus, the mass of arsenic present in 5.0 g of orpiment is, 1.5 grams.