Answer:
The pH is equal to 4.41
Step-by-step explanation:
Since HClO is a weak acid, its dissociation in aqueous medium is:
HClO ⇄ ClO- + H+
start: 0.05 0 0
change -x +x +x
balance 0.05-x x x
As it is a weak acid it dissociates very little, in its ClO- and H + ions, so the change is negative, where x is a degree of dissociation.
the acidity constant when equilibrium is reached is equal to:
![Ka=([ClO-]*[H+])/([HClO])=(x*x)/(0.05-x)=3x10^(-8)](https://img.qammunity.org/2021/formulas/chemistry/college/iqp0jbftjmdsglglh90spf6k68j2n97q0d.png)
The 0.05-x fraction can be approximated to 0.05, because the ionized fraction (x) is very small, therefore we have:
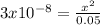
clearing the x and calculating its value we have:
![x=3.87x10^(-5)=[H+]=[ClO-]](https://img.qammunity.org/2021/formulas/chemistry/college/6krrecros16i9v7s1mbircbn6iygosa823.png)
the pH can be calculated by:
![pH=-log[H+]=-log[3.87x10^(-5)]=4.41](https://img.qammunity.org/2021/formulas/chemistry/college/hdb1t896g2nzu0z2xacnw69siobfk3x9au.png)