Answer : The moles of given compound is, 0.064 mole
Explanation : Given,
Mass of given compound = 40 g
Atomic mass of X = 50 amu
Atomic mass of Y = 45 amu
Atomic mass of Z = 10 amu
First we have to calculate the molar mass of given compound.
The given compound formula is,
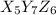
Molar mass of
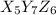 = (5 × Atomic mass of X) + (7 × Atomic mass of Y) + (6 × Atomic mass of Z)
= (5 × Atomic mass of X) + (7 × Atomic mass of Y) + (6 × Atomic mass of Z)
Molar mass of
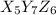 = (5 × 50) + (7 × 45) + (6 × 10) = 625 g/mol
= (5 × 50) + (7 × 45) + (6 × 10) = 625 g/mol
Now we have to calculate the moles of given compound.
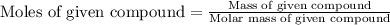
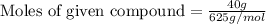
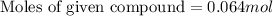
Thus, the moles of given compound is, 0.064 mole