Answer:
- 602 mg of CO₂ and 94.8 mg of H₂O
Step-by-step explanation:
The yield is measured by the amount of each product produced by the reaction.
The chemical formula of fluorene is C₁₃H₁₀, and its molar mass is 166.223 g/mol.
The oxidation, also know as combustion, of this hydrocarbon is represented by the following balanced chemical equation:
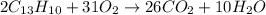
To calculate the yield follow these steps:
1. Mole ratio
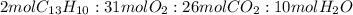
2. Convert 175mg of fluorene to number of moles
- Number of moles = mass in grams / molar mass
3. Set a proportion for each product of the reaction
a) For CO₂
i) number of moles
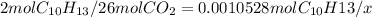
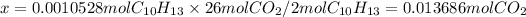
ii) mass in grams
The molar mass of CO₂ is 44.01g/mol
- mass = number of moles × molar mass
- mass = 0.013686 moles × 44.01 g/mol = 0.602 g = 602mg
b) For H₂O
i) number of moles
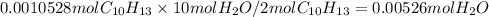
ii) mass in grams
The molar mass of H₂O is 18.015g/mol
- mass = number of moles × molar mass
- mass = 0.00526 moles × 18.015 g/mol = 0.0948mg = 94.8 mg