Answer:
Specific heat of brass is 0.40 J g⁻¹ °C⁻¹ .
Step-by-step explanation:
Given :
Mass of brass, m₁ = 440 g
Temperature of brass, T₁ = 97° C
Mass of water, m₂ = 350 g
Temperature of water, T₂ = 23° C
Specific heat of water, C₂ = 4.18 J g⁻¹ °C⁻¹
Equilibrium temperature, T = 31° C
Let C₁ be the specific heat of brass.
Heat loss by brass = Heat gain by water
m₁ x C₁ x ( T₁ -T ) = m₂ x C₂ x ( T - T₁ )
Substitute the suitable values in above equation.
440 x C₁ x (97 - 31) = 350 x 4.18 x (31 - 23)
C₁ =
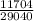
C₁ = 0.40 J g⁻¹ °C⁻¹