Answer: The cell voltage of the given cell is 2.01
Step-by-step explanation:
The given chemical cell equation follows:
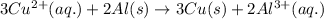
Oxidation half reaction:
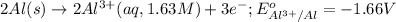 ( × 2)
( × 2)
Reduction half reaction:
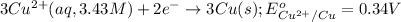 ( × 3)
( × 3)
Oxidation reaction occurs at anode and reduction reaction occurs at cathode.
To calculate the
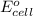 of the reaction, we use the equation:
of the reaction, we use the equation:
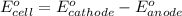
Putting values in above equation, we get:
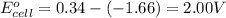
To calculate the EMF of the cell, we use the Nernst equation, which is:
![E_(cell)=E^o_(cell)-(2.303RT)/(nF)\log ([Al^(3+)]^2)/([Cu^(2+)]^3)](https://img.qammunity.org/2021/formulas/chemistry/college/zu2hbxs1z58kumrs45krz1arlkfl6j0c62.png)
where,
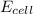 = electrode potential of the cell = ? V
= electrode potential of the cell = ? V
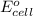 = standard electrode potential of the cell = +2.00 V
= standard electrode potential of the cell = +2.00 V
R = Gas constant = 8.314 J/mol.K
T = temperature =
![42^oC=[42+273]K=315K](https://img.qammunity.org/2021/formulas/chemistry/college/y02z296wo03t4xb5nqv4jhyjq7nvy3790h.png)
F = Faraday's constant = 96500
n = number of electrons exchanged = 6
![[Cu^(2+)]=3.43M](https://img.qammunity.org/2021/formulas/chemistry/college/stcnlo9n3x6f4m77b82lj316jhpu1dfi98.png)
![[Al^(3+)]=1.63M](https://img.qammunity.org/2021/formulas/chemistry/college/4k6beftczb7danwfnzpvvz8g9lfd4sf51k.png)
Putting values in above equation, we get:
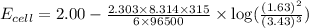
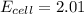
Hence, the cell voltage of the given cell is 2.01