The question is incomplete, here is the complete question:
A scuba diver is at a depth of 355 m, where the pressure is 36.5 atm.
What should be the mole fraction of
 in the gas mixture the diver breathes in order to have the same partial pressure of oxygen in his lungs as he would at sea level? Note that the mole fraction of oxygen at sea level is 0.209.
in the gas mixture the diver breathes in order to have the same partial pressure of oxygen in his lungs as he would at sea level? Note that the mole fraction of oxygen at sea level is 0.209.
Answer: The mole fraction of oxygen in the gas mixture is 0.00573
Step-by-step explanation:
To calculate the partial pressure, we use the equation given by Raoult's law, which is:
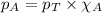 ........(1)
........(1)
where,
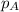 = partial pressure of oxygen at sea level = ?
= partial pressure of oxygen at sea level = ?
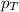 = total pressure at sea level = 1.00 atm
= total pressure at sea level = 1.00 atm
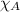 = mole fraction of oxygen at sea level = 0.209
= mole fraction of oxygen at sea level = 0.209
Putting values in equation 1, we get:

As, partial pressure of the oxygen in the diver's lungs is equal to the partial pressure of oxygen at sea level
We are given:
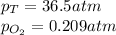
Putting values in equation 1, we get:
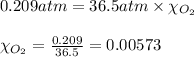
Hence, the mole fraction of oxygen in the gas mixture is 0.00573