The question is incomplete , complete question is:
Hydrofluoric acid and water react to form fluoride anion and hydronium cation, like this:
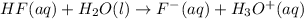
At a certain temperature, a chemist finds that a reaction vessel 5.6 L containing an aqueous solution of hydrofluoric acid, water, fluoride anion, and hydronium cation at equilibrium has the following composition: compound amount
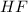 = 1.62 g
= 1.62 g
 = 516 g
= 516 g
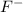 = 0.163 g
= 0.163 g
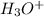 = 0.110 g
= 0.110 g
Calculate the value of the equilibrium constant for this reaction. Round your answer to significant digits.
Answer:
The value of equilibrium constant of the reaction is
 .
.
Step-by-step explanation:
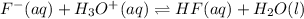
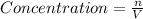
Where :
n = moles of compound
V = Volume of the solution L
Moles of HF =
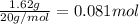
Volume of the solution in the vessel = V = 5.6 L
![[HF]=(0.081 mol)/(5.6 L)=0.01446 M](https://img.qammunity.org/2021/formulas/chemistry/college/gza2n78t2r9015b8nteskrwitwpkjbnja9.png)
Moles of
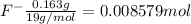
Volume of the solution in the vessel = V = 5.6 L
![[F^-]=(0.008579 mol)/(5.6 L)=0.001532 M](https://img.qammunity.org/2021/formulas/chemistry/college/eymmetn59ubfpqf6xejonvsb41tn22nma1.png)
Moles of
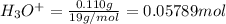
Volume of the solution in the vessel = V = 5.6 L
![[H_3O^+]=(0.05789 mol)/(5.6 L)=0.001034 M](https://img.qammunity.org/2021/formulas/chemistry/college/zdavjqrtpkyx9ea8z861qm55h7ez0q4j58.png)
An equilibrium expression is given as;
![K_c=([F^-][H_3O^+])/([HF])](https://img.qammunity.org/2021/formulas/chemistry/college/1aqf6hfhn517av9a4o0u786a2t0u46bv7y.png)
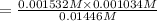
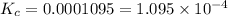
The value of equilibrium constant of the reaction is
 .
.