Answer : The concentrations of A, B, and C at equilibrium is, 0.11 M, 0.21 M and 0.19 M respectively.
Explanation :
The relation between the equilibrium constant and standard Gibbs, free energy is:
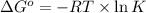
where,
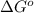 = standard Gibbs, free energy = -5.43 kJ/mol = -5430 J/mol
= standard Gibbs, free energy = -5.43 kJ/mol = -5430 J/mol
R = gas constant = 8.314 J/mol.K
T = temperature =
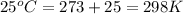
K = equilibrium constant = ?
Now put all the given values in the above relation, we get:
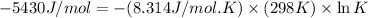
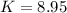
Now we have to calculate the concentrations of A, B, and C at equilibrium.
The balanced equilibrium chemical reaction is:
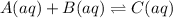
Initial conc. 0.30 0.40 0
At eqm. (0.30-x) (0.40-x) x
The expression for equilibrium constant for this reaction is,
![K=([C])/([A][B])](https://img.qammunity.org/2021/formulas/physics/college/q05lj9ssm8gu6e6a1stkwjsleo2qlpyt01.png)
Now put all the given values in this expression, we get:
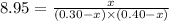
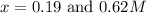
As we know that the concentration at equilibrium can not be more than initial concentration. So, neglecting the value of x = 0.62 M
The value of 'x' will be, 0.19 M
Thus, the concentrations of A at equilibrium = (0.30-x) = (0.30-0.19) = 0.11 M
The concentrations of B at equilibrium = (0.40-x) = (0.40-0.19) = 0.21 M
The concentrations of C at equilibrium = x = 0.19 M