Answer:
The answer is 26.45%
Step-by-step explanation:
Given:
Mass of solute = 5 g
Mass of solvent = 13.9 g
Required
Mass percent = ?
Solution
Mass percent is calculated by
Mass percent = (Mass of solute / mass of solution) x 100
Mass percent = mass of solute / (mass of solute + mass of solution) x 100
Mass percent =
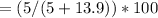
Mass percent = 26.45 %