Answer : The molality of solution is, 0.866 mole/kg
Explanation :
The relation between the molarity, molality and the density of the solution is,
![d=M[(1)/(m)+(M_b)/(1000)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/7j5nkokv1wya72zqm94lw340z3lgegyu0b.png)
where,
d = density of solution = 1.0052 g/ml
m = molality of solution = ?
M = molarity of solution = 0.828 M = 0.828 mol/L
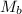 = molar mass of solute (acetic acid) = 60.05 g/mole
= molar mass of solute (acetic acid) = 60.05 g/mole
Now put all the given values in the above formula, we get the molality of the solution.
![1.0052g/ml=0.828mol/L* [(1)/(m)+(60.05g/mole)/(1000)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/d0688e7597r520a26p98w0phdrdahjqc8v.png)
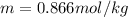
Therefore, the molality of solution is, 0.866 mole/kg