Answer:
The vapor pressure of ethanol at 60.61 °C is 327.56 mmHg
Step-by-step explanation:
using Clausius-Clapeyron equation
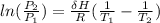
where;
ΔH is the enthalpy of vaporization of ethanol = 39.3 kJ/mol
R is ideal gas constant = 8.314 J/mol.K
P₁ is the initial pressure of ethanol at T₁ = 1.00 × 10² mmHg
P₂ is the final pressure of ethanol at T₂ = ?
T₁ is the initial temperature = 34.9°C = 307.9 K
T₂ is the final temperature = 60.61°C = 333.61 K
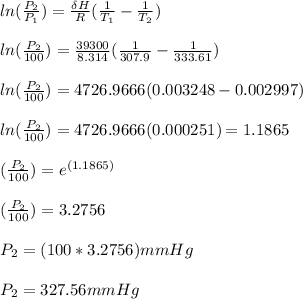
Therefore, the vapor pressure of ethanol at 60.61 °C is 327.56 mmHg