The question is incomplete, here is the complete question:
An analytical chemist is titrating 182.2 mL of a 1.200 M solution of nitrous acid with a solution of 0.8400 M KOH. The
 of nitrous acid is 3.35. Calculate the pH of the acid solution after the chemist has added 46.44 mL of the KOH solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of solution added. Round your answer to 2 decimal places.
of nitrous acid is 3.35. Calculate the pH of the acid solution after the chemist has added 46.44 mL of the KOH solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of solution added. Round your answer to 2 decimal places.
Answer: The pH of the solution is 2.69
Step-by-step explanation:
To calculate the number of moles for given molarity, we use the equation:
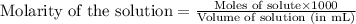
Molarity of nitrous acid = 1.200 M
Volume of solution = 182.2 mL
Putting values in above equation, we get:
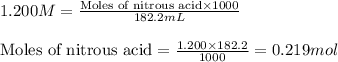
Molarity of KOH = 0.8400 M
Volume of solution = 46.44 mL
Putting values in above equation, we get:

The chemical reaction for KOH and nitrous acid follows the equation:

Initial: 0.219 0.039
Final: 0.18 - 0.039
Volume of solution = 182.2 + 46.44 = 228.64 mL = 0.22864 L (Conversion factor: 1 L = 1000 mL)
To calculate the pH of acidic buffer, we use the equation given by Henderson Hasselbalch:
![pH=pK_a+\log(([salt])/([acid]))](https://img.qammunity.org/2021/formulas/biology/college/6usxe642bp3w274zbcv30her0kcessu95f.png)
![pH=pK_a+\log(([KNO_2])/([HNO_2]))](https://img.qammunity.org/2021/formulas/chemistry/college/naf8rdfdyo28lrde1infrgso4herc70uml.png)
We are given:
 = negative logarithm of acid dissociation constant of nitrous acid = 3.35
= negative logarithm of acid dissociation constant of nitrous acid = 3.35
![[KNO_2]=(0.039)/(0.22864)](https://img.qammunity.org/2021/formulas/chemistry/college/d4laf2o3ynfimopuh0sngpl2hmmvqe643i.png)
![[HNO_2]=(0.18)/(0.22864)](https://img.qammunity.org/2021/formulas/chemistry/college/1t44luxenzrad3347eoywl7w2gfiioawxc.png)
pH = ?
Putting values in above equation, we get:
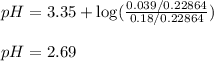
Hence, the pH of the solution is 2.69