This is an incomplete question, here is a complete question.
Here are the solubilities of a particular solute of two different temperatures:
Temperature (°C) Solubility (g/100 g H₂O)
20.0 28.4
30.0 76.5
Suppose that you have made a saturated solution of this solute using 85.0 g of water at 20.0 °C. How much more solute can you add if the temperature is increased to 30.0 °C. (PLEASE explain in detail & answer in grams)
Answer : The more amount of solute added can be, 40.885 grams.
Explanation :
First we have to calculate the amount of solute at 20.0 °C.
As, 100 g of water contains amount of solute = 28.4 g
So, 85.0 g of water contains amount of solute =
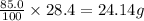
Now we have to calculate the amount of solute at 30.0 °C.
As, 100 g of water contains amount of solute = 76.5 g
So, 85.0 g of water contains amount of solute =
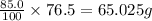
Now we have to calculate the more amount of solute can be added.
Amount of solute added = 65.025 g - 24.14 g
Amount of solute added = 40.885 g
Thus, the more amount of solute added can be, 40.885 grams.