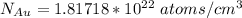Answer:
The Number of gold atoms are =
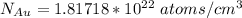
Step-by-step explanation:
The formula we are going to use is:
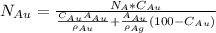
Where:
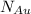 are number of gold atoms.
are number of gold atoms.
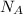 is Avogadro Number.
is Avogadro Number.
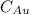 is the amount of gold.
is the amount of gold.
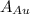 is the atomic weight of gold.
is the atomic weight of gold.
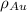 is the density of gold.
is the density of gold.
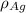 is the density of silver.
is the density of silver.
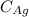 is the amount of silver.
is the amount of silver.
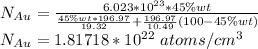
The Number of gold atoms are =