Answer:
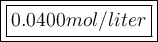
Step-by-step explanation:
Knowing that you have 64.92 grams of Hg(NO₃)₂ to make 5.00 liters of solution, you can calcualte the molarity of the solution.
Molarity is a measure of concentration, defined as the number of moles of solute per liter of soluiton. Mathematically:
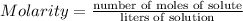
Then, first you must calculate the number of moles of solute. The formula is:
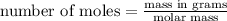
You can either calculate the molar mass of the compound using the chemical formula or search it in the internet.
The molar mass of Hg(NO₃)₂ is found to be 324.7 g/mol.
Now you have everything to calculate the molarity of the solution: