Answer:
47,9 kJ/g
Step-by-step explanation:
The enthalpy of combustion is the change in energy due the reaction of combustion of n-octane. If the enthalpy is -4,79x10⁷ J/kg And knowing (1000J = 1kJ) (1000g = 1kg). The enthalpy of combustion expressed in kJ/g is:
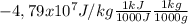 = 47,9kJ/g
= 47,9kJ/g
I hope it helps!