Answer: The freezing point of solution is -0.974°C
Step-by-step explanation:
- To calculate the concentration of solute, we use the equation for osmotic pressure, which is:
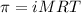
where,
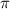 = osmotic pressure of the solution = 12.1 atm
= osmotic pressure of the solution = 12.1 atm
i = Van't hoff factor = 1 (for non-electrolytes)
M = molarity of solute = ?
R = Gas constant =
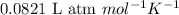
T = temperature of the solution = 298 K
Putting values in above equation, we get:
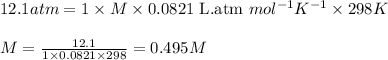
This means that 0.495 moles of glucose is present in 1 L or 1000 mL of solution
- To calculate the mass of solution, we use the equation:
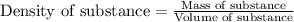
Density of solution = 1.034 g/mL
Volume of solution = 1000 mL
Putting values in above equation, we get:
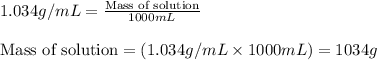
- To calculate the number of moles, we use the equation:

Moles of glucose = 0.495 moles
Molar mass of glucose = 180.16 g/mol
Putting values in above equation, we get:
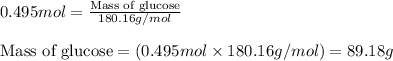
Depression in freezing point is defined as the difference in the freezing point of pure solution and freezing point of solution.
- The equation used to calculate depression in freezing point follows:
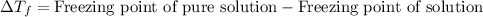
To calculate the depression in freezing point, we use the equation:
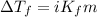
Or,
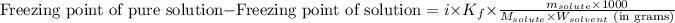
where,
Freezing point of pure solution = 0°C
i = Vant hoff factor = 1 (For non-electrolytes)
 = molal freezing point elevation constant = 1.86°C/m
= molal freezing point elevation constant = 1.86°C/m
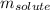 = Given mass of solute (glucose) = 89.18 g
= Given mass of solute (glucose) = 89.18 g
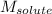 = Molar mass of solute (glucose) = 180.16 g/mol
= Molar mass of solute (glucose) = 180.16 g/mol
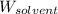 = Mass of solvent (water) = [1034 - 89.18] g = 944.82 g
= Mass of solvent (water) = [1034 - 89.18] g = 944.82 g
Putting values in above equation, we get:
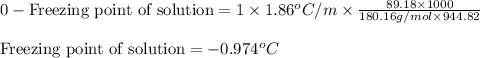
Hence, the freezing point of solution is -0.974°C