Answer:
The exact time when the sample was taken is = 0.4167337 hr
Step-by-step explanation:
The diagram of a sketch of the tank is shown on the first uploaded image
Let A denote the first inlet
Let B denote the second inlet
Let C denote the single outflow from the tank
From the question we are given that the diameter of A is = 1 cm = 0.01 m
Area of A is =
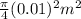
=
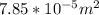
Velocity of liquid through A = 0.2 m/s
The rate at which the liquid would flow through the first inlet in terms of volume =
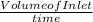 =
=
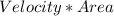 i.e is
i.e is
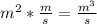
=
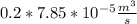
The rate at which the liquid would flow through the first inlet in terms of mass of the liquid = mass of liquid × the rate of flow in terms of volume
=
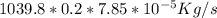
= 0.016324
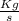
From the question the diameter of B = 2 cm = 0.02 m
Area of B =
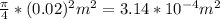
Velocity of liquid through B = 0.01 m/s
The rate at which the liquid would flow through the first inlet in terms of volume =
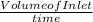 =
=
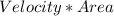 i.e is
i.e is
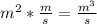
=
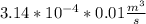
The rate at which the liquid would flow through the second inlet in terms of mass of the liquid = mass of liquid × the rate of flow in terms of volume
=
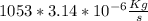
= 0.00330642
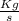
From the question The flow rate in term of volume of the outflow at the time of measurement is given as = 0.5 L/s
And also from the question the mass of potassium chloride at the time of measurement is given as 13 g/L
So The rate at which the liquid would flow through the outflow in terms of mass of the liquid = mass of liquid × the rate of flow in terms of volume
=
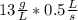
=
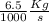 Note (1 Kg = 1000 g)
Note (1 Kg = 1000 g)
= 0.0065 kg/s
Considering potassium chloride
Let denote the rate at which liquid flows in terms of mass as as
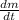 i.e change in mass with respect to time hence
i.e change in mass with respect to time hence
Input(in terms of mass flow ) - output(in terms of mass flow ) = Accumulation in the Tank(in terms of mass flow )
(0.016324 + 0.00330642) - 0.0065 =
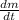
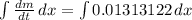
=> 0.01313122 t = (m -
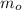 )
)
From the question (m -
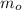 ) is given as = 19.7 Kg
) is given as = 19.7 Kg
Hence the time when the sample was taken is given as
0.01313122 t = 19.7 Kg
=> t = 1500.2414 sec
t = .4167337 hours (1 hour = 3600 seconds)