This is an incomplete question, here is a complete question.
A chemistry graduate student is given 500 mL of a 0.20 M pyridine C₅H₅N solution. Pyridine is a weak base with Kb = 1.7 × 10⁻⁹. What mass of C₅H₅NHCl should the student dissolve in the C₅H₅N solution to turn it into a buffer with pH = 4.76 ?
You may assume that the volume of the solution doesn't change when the C₅H₅NHCl is dissolved in it. Be sure your answer has a unit symbol, and round it to 2 significant digits.
Answer : The mass of C₅H₅NHCl is, 34 grams.
Explanation :
First we have to calculate the value of
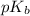 .
.
The expression used for the calculation of
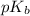 is,
is,
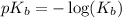
Now put the value of
 in this expression, we get:
in this expression, we get:
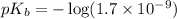
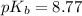
Now we have to calculate the value of

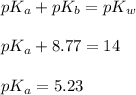
Now we have to calculate the concentration of C₅H₅NHCl.
Using Henderson Hesselbach equation :
![pH=pK_a+\log ([Salt])/([Acid])](https://img.qammunity.org/2021/formulas/biology/college/z944fnahhldpjolfrvealc6q9baj5h69q3.png)
![pH=pK_a+\log ([C_5H_5N])/([C_5H_5NHCl])](https://img.qammunity.org/2021/formulas/chemistry/college/rkq47xam4pqozqg0l4aft4c4pt97fa1l1r.png)
Now put all the given values in this expression, we get:
![4.76=5.23+\log ((0.20)/([C_5H_5NHCl]))](https://img.qammunity.org/2021/formulas/chemistry/college/1afu7ks71fd1man6kozqqu9xfv9ox6zw2b.png)
![[C_5H_5NHCl]=0.59M](https://img.qammunity.org/2021/formulas/chemistry/college/suiuxivvc96ypz27x2oearnhix100uldkz.png)
Now we have to calculate the mass of C₅H₅NHCl.
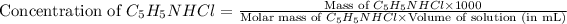
Now put all the given values in this formula, we get:
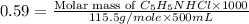
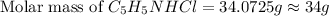
Thus, the mass of C₅H₅NHCl is, 34 grams.