Answer : The [α] for the solution is, -118.8
Explanation :
Enantiomeric excess : It is defined as the difference between the percentage major enantiomer and the percentage minor enantiomer.
Mathematically,
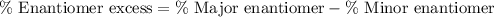
Given:
% major enantiomer = 86 %
% minor enantiomer = 14 %
Putting values in above equation, we get:
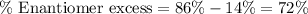
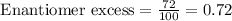
Now we have to calculate the [α] for the solution.
![[\alpha]=\text{Enantiomer excess}* [\alpha]_(Pure)](https://img.qammunity.org/2021/formulas/chemistry/college/j38xnghjoa3x89op5axvmq5y4y67wh92if.png)
![[\alpha]=0.72* -165](https://img.qammunity.org/2021/formulas/chemistry/college/hmp2520hd69tds4ms9g13g3zzf45ql0940.png)
![[\alpha]=-118.8](https://img.qammunity.org/2021/formulas/chemistry/college/5fgfjx6hw5xij4ujggol9anf2ict0ppvnu.png)
Thus, the [α] for the solution is, -118.8