Step-by-step explanation:
It is known that molality is the number of moles present in kg of solution.
Mathematically, Molality =
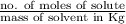
The given data is as follows.
Molar mass of ammonia = 17 g/mol
Concentration = 1.002 mg/L =
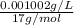
=
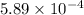 mol/L
mol/L
Also, density =
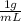 = 1 kg/L
= 1 kg/L
Therefore, molality will be calculated as follows.
Molality =
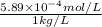
=
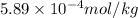
And,
Molar mass of nitrite = 46 g/mol
Concentration = 0.387 mg/L =
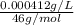
=
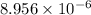 mol/L
mol/L
And, density =
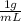 = 1 kg/L
= 1 kg/L
Hence, molality =
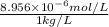
=
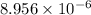 mol/kg
mol/kg
Now, Molar mass of nitarte = 62 g/mol
Concentration = 1352.2 mg/L
=
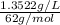
= 0.02181 mol/L
Also, density =
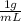 = 1 kg/L
= 1 kg/L
Hence, molality will be calculated as follows.
Molality =
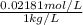
= 0.02181 mol/kg
Therefore, molality of given species is
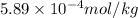 for ammonia,
for ammonia,
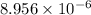 mol/kg for nitrite, and 0.02181 mol/kg for nitrate ion.
mol/kg for nitrite, and 0.02181 mol/kg for nitrate ion.