Answer:
1.Cp₁ = 1.2 J/g.⁰C
Step-by-step explanation:
For new material:
m₁ = 25 g
T₁ = 80⁰C
specific heat of water = Cp₁
For water :
m₂ = 100 g
T₂ = 20⁰C
The final temperature T=24⁰C
We know that specific heat of water Cp₂ = 4.187 kJ/kg.K
The heat lost new material = Heat gain by Water
m₁ Cp₁ ( T₁ - T ) = m₂ Cp₂ (T- T₂)
25 x Cp₁ (80- 24 ) = 100 x 4.817 (24 - 20 )
Cp₁ x 56 = 4 x 4.187 x 4
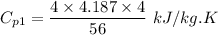
Cp₁ = 1.19 kJ/kg.K
Cp₁ = 1.2 J/g.⁰C