Answer:
548s = t
Step-by-step explanation:
A first-order reaction has as general equation:
![ln ([A]_t)/([A]_0) = -kt](https://img.qammunity.org/2021/formulas/chemistry/college/183iotaszh8cgg12ycseihyu7vrzemi764.png)
Where
![[A]_t](https://img.qammunity.org/2021/formulas/chemistry/college/yxb5obac4tb5x24xab182isglk8wsoulak.png) is the concentration of the reactant in the time t,
is the concentration of the reactant in the time t,
![[A]_0](https://img.qammunity.org/2021/formulas/chemistry/college/9ieb8lxqiipbpacc2ob6pjacwjf55c1wel.png) is the initial concentration of the reactant, k is rate constant and t is time. Replacing with values of the problem:
is the initial concentration of the reactant, k is rate constant and t is time. Replacing with values of the problem:
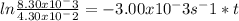
-1.65 = -3.00x10⁻³s⁻¹×t
548s = t
I hope it helps!