Answer : The mass of oxygen per gram of sulfur for sulfur dioxide and sulfur trioxide is, 0.997 g and 1.5 g respectively.
Explanation : Given,
Mass of oxygen in sulfur dioxide = 3.49 g
Mass of sulfur in sulfur dioxide = 3.50 g
Mass of oxygen in sulfur trioxide = 9.00 g
Mass of sulfur in sulfur trioxide = 6.00 g
Now we have to calculate the mass of oxygen per gram of sulfur for sulfur dioxide and sulfur trioxide.
Mass of oxygen per gram of sulfur for sulfur dioxide =
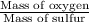
Mass of oxygen per gram of sulfur for sulfur dioxide =
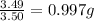
and,
Mass of oxygen per gram of sulfur for sulfur trioxide =
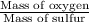
Mass of oxygen per gram of sulfur for sulfur trioxide =
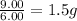
Thus, the mass of oxygen per gram of sulfur for sulfur dioxide and sulfur trioxide is, 0.997 g and 1.5 g respectively.