Answer : The mass of one copper atom is, [tex1.06\times 10^{-25}kg[/tex]
Explanation : Given,
Mass of one mole of copper = 64 g
First we have to convert the mass of copper from gram to kilogram.
Conversion used :
1 kg = 1000 g
or,
1 g = 0.001 kg
So, mass of copper = 64 g = 0.064 kg
Now we have to calculate the mass of one copper atom.
As,
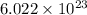 atoms of mass of copper = 0.064 kg
atoms of mass of copper = 0.064 kg
So, 1 atoms of mass of copper =
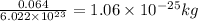
Thus, the mass of one copper atom is, [tex1.06\times 10^{-25}kg[/tex]