Answer: The amount remained after 151 seconds are 0.041 moles
Step-by-step explanation:
All the radioactive reactions follows first order kinetics.
Rate law expression for first order kinetics is given by the equation:
![k=(2.303)/(t)\log([A_o])/([A])](https://img.qammunity.org/2021/formulas/chemistry/college/bbi6c2ny1tf8wlzntta3i570f6pal714ld.png)
where,
k = rate constant =
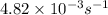
t = time taken for decay process = 151 sec
![[A_o]](https://img.qammunity.org/2021/formulas/physics/college/3jrctnxyrdjmiz9ngr0s6o9r3hdvpo6qhe.png) = initial amount of the reactant = 0.085 moles
= initial amount of the reactant = 0.085 moles
[A] = amount left after decay process = ?
Putting values in above equation, we get:
![4.82* 10^(-3)=(2.303)/(151)\log(0.085)/([A])](https://img.qammunity.org/2021/formulas/chemistry/college/to8jbdbr27tm2l35gi8m00zjdhinwzkngu.png)
![[A]=0.041moles](https://img.qammunity.org/2021/formulas/chemistry/college/mjc4gm49zasbtw4zviwzf1vgz6qvqhooc2.png)
Hence, the amount remained after 151 seconds are 0.041 moles