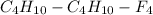The given formula of
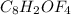 can be written as
can be written as
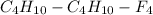
Step-by-step explanation:
Empirical formula is one of the way of representing the structural arrangement of any molecule. With this formula, we can guess the nearest atom or element in a heterogeneous molecules. Mostly the empirical and molecular formula differs for organic compounds.
As in organic compounds consists of chain of carbon and hydrogen elements. So while arranging them in a relation, we should remember that carbon should contain 4 nearby atoms and hydrogen can share only one electron to its nearby atom. Thus, a carbon can have three hydrogen and one carbon as neighbouring element.
So the given formula of
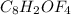 can be written as
can be written as