Step-by-step explanation:
As the given chemical reaction equation is as follows.
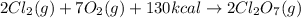
Also, it is given that for 2 moles the energy required is 130 kcal. This means that energy required for 1 mole is calculated as follows.
1 mole =
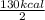
= 65 kcal
Hence, energy required for 7 moles will be calculated as follows.
Energy required =
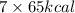
= 455 kcal
Thus, we can conclude that energy required to produce 7.00 mol
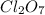 on the basis of given reaction is 455 kcal.
on the basis of given reaction is 455 kcal.